Navigating the intricate landscape of US Pricing and Reimbursement Dossiers requires a deep understanding and strategic approach. Justin Stindt Consultants, a distinguished global consulting agency, offers unparalleled expertise in the realm of AMCP and ICER dossiers, essential tools for formulary decision-making in the US. As pharmaceutical and biotech companies aim for successful market entry, the significance of a meticulously crafted dossier cannot be understated. Our international team of experts is adept at synthesizing vast amounts of data into coherent, impactful dossiers that resonate with healthcare decision-makers. Our agency is committed to ensuring your product’s value proposition is compellingly presented, optimizing its market access potential. Partner with Justin Stindt Consultants to develop US Pricing & Reimbursement dossiers enabling coverage across the world’s largest market.
Understanding AMCP and ICER Dossiers for Healthcare Pricing & Reimbursement in the USA: A Comprehensive Overview
The Academy of Managed Care Pharmacy (AMCP) Format for Formulary Submissions (AMCP Format) is a framework that provides guidance to pharmaceutical and biotech manufacturers on the development of dossiers for formulary and coverage consideration. The AMCP Format is designed to be comprehensive and considers all sources of information that are relevant to formulary decision-making by health care decision makers (HCDMs).
Justin Stindt Consultants is an agency specialized in AMCP dossiers. Our international and United-States experienced team of Consultants and Experts will help you to articulate the added benefit of your product in the AMCP dossier.

Crafting Comprehensive Dossiers for HCDMs: Product Profiles, Clinical Evidence, and Economic Analysis for Informed Drug Formulary Decisions
The dossier itself is a comprehensive document that provides all of the information that HCDMs need to make a formulary and coverage decision for a new drug or biologic. The dossier is typically divided into the following sections:
- Product Profile: This section provides an overview of the product, including its indication, dosage, safety, and efficacy.
- Clinical Evidence: This section provides a review of the clinical evidence for the product, including randomized controlled trials, observational studies, and real-world data.
- Economic Analysis: This section provides an economic evaluation of the product, including a cost-effectiveness analysis and a budget impact analysis.
- Additional Information: This section provides any additional information that is relevant to formulary decision-making, such as patient-reported outcomes, pharmacovigilance data, and payer-specific information.
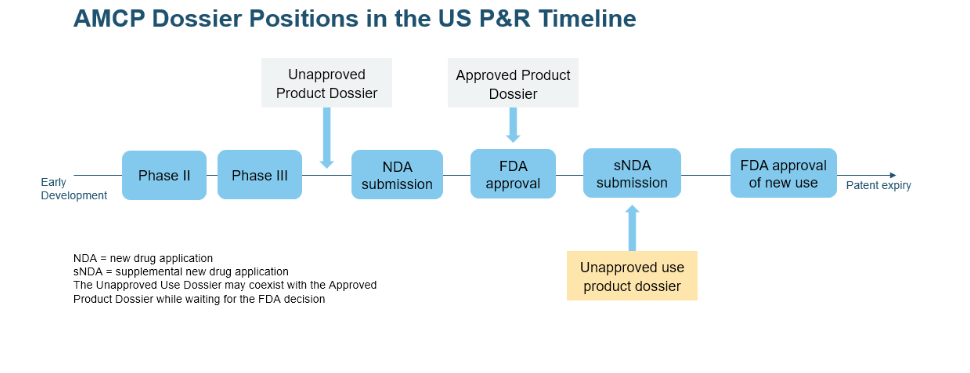
Types of AMCP Dossiers: unapproved product dossiers, approved product dossiers, and unapproved use dossiers
The AMCP Format defines three types of dossiers: unapproved product dossiers, approved product dossiers, and unapproved use dossiers. This differentiation is necessary because the information requirements for each type of dossier are different and because it allows HCDMs to make informed decisions about whether or not to cover a product for a particular indication. For example, an HCDM may be more likely to cover an unapproved product for a serious or life-threatening condition, even if the product has not yet been approved by the FDA.
| Dossier Type | Information Requirements | Purpose |
|---|---|---|
| Unapproved Product Dossier | Safety, efficacy, and potential clinical utility | To convey details about a product not yet approved by the FDA |
| Approved Product Dossier | Clinical evidence, economic evaluation, and value proposition | To share information about an FDA-approved product |
| Unapproved Use Dossier | Safety, efficacy, and potential clinical utility for an unapproved use | To communicate data about an unapproved use of an approved product |
ICER and its role in US Market Access
In addition to the AMCP Dossiers, manufacturers may consider submitting documentation to the Institute for Clinical and Economic Review (ICER). ICER is an independent, non-profit research organization that conducts health technology assessments (HTAs) for payers in the United States. While ICER’s assessments do not have direct regulatory authority, they are influential in shaping discussions around drug pricing, reimbursement decisions, and market access for medicines in the United States. Insurers, pharmacy benefit managers, and other payers may consider ICER’s evaluations when making coverage and pricing decisions.
Justin Stindt Consultants, Your Expert Consulting Agency for US Pricing & Reimbursement Dossiers

Justin Stindt Consultants’ Expertise for AMCP and ICER Dossiers
Our commitment lies in delivering dossiers that excel in written excellence, technical precision, and the art of weaving clear, captivating narratives. Our goal, as a consulting agency, is to ensure that each dossier forms a cohesive, integrated, evidence-based story, moving away from a mere assortment of distinct clinical and health-economic elements typically found in an evidence package.
Justin Stindt Consultants is your global agency with the right experts in supporting the preparation of AMCP and ICER dossiers. Our services include (not limited to):
- Comprehensive project planning with clear timelines and milestones
- Strategic review of drug positioning
- Dossier writing
- Dossier validation with Key Opinion Leaders and payers / HCDMs
- Submission process guidance and support
- Strategic advice and participation in contracting
- Economic modelling
- Support in commenting ICER Draft Scoping Document, Draft Evidence Report and Oral Comment at the Public Meeting
- Effective project management and communication throughout the process
At Justin Stindt Consultants, our team of experts has extensive experience in providing our clients with tailored support to provide access to market and patients in the US as soon as possible at sustainable pricing and reimbursement conditions. If you are looking for a vendor to support your US dossiers, select a service provider that will go the extra mile to enable coverage for your product.
Let Justin Stindt Consultants be your trusted agency partner in unlocking pricing & reimbursement (P&R) opportunities for your product in the USA.
Contact us today to learn how our firm can tailor our services to meet your unique needs and make your pricing & reimbursement goals a reality.
Meet one of our Pricing and Reimbursement dossiers US experts

Get in touch with our expert
CLIENT TESTIMONIALS