In the multifaceted landscape of Pricing and Reimbursement Dossiers Germany, Justin Stindt Consultants stands as a proficient consulting agency dedicated to guiding companies through the intricate German reimbursement ecosystem. As a global consulting agency, our firm is backed by an international team of experts well-versed in market access strategies within the Federal Republic of Germany. Our consultants and specialists are committed to ensuring that pharmaceutical interventions align with the stringent regulations of the German market. Recognized as a trusted contractor, our agency’s expertise is essential for those aiming to comprehend and deliver a German Benefit Assessment (AMNOG) dossier resonating with G-BA and IQWiG. Collaborate with Justin Stindt Consultants to harness our comprehensive insights and achieve sustainable pricing and reimbursement outcomes in Germany.
Understanding the Impact of AMNOG Reform on Healthcare Pricing & Reimbursement Dossiers in Germany
German Benefit Assessment (AMNOG) Dossier
The AMNOG reform of 2011 has introduced more scrutiny towards new drug approvals and thus impacted pricing potential. The benefit assessment dossier (“AMNOG dossier”) is a critical part of the added benefit assessment and it is fundamental to submit a dossier that is complete, accurate, and oriented towards the requirements of the Federal Joint Committee (Gemeinsamer Bundesausschuss, G-BA) and the Institut für Qualität und Wirtschaftlichkeit im Gesundheitswesen (IQWiG).
Justin Stindt Consultants is an agency specialized in German benefit assessment dossiers. Our experienced team of Consultants and Experts will help you to articulate the added benefit of your product in the AMNOG dossier.

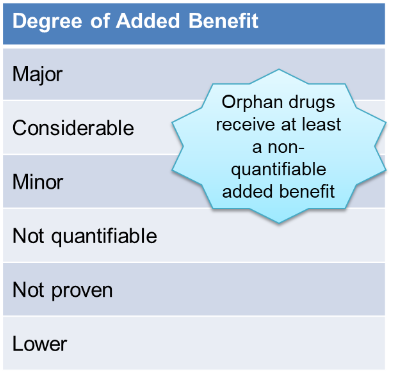
The G-BA bases its Added Benefit assessment on the dossier submitted by the manufacturer. Most recent assessments fall within the categories of minor or not quantifiable added benefit, which can lead to challenging pricing discussions with the GKV-Spitzenverband.
Given how critical the dossier and its contents are to this decision, it is important to submit a dossier that is complete, accurate, and oriented towards the requirements of the G-BA and IQWiG. The optimal way to ensure that the information in the dossier matches the requirements of the G-BA is to have an early advice meeting directly with the G-BA. For more information visit our Health Technology Appraisal scientific advice discussions – Germany.
Although Germany remains an attractive market for pharma, biotech and MedTech, where premium pricing is possible, if G-BA requirements are not met, GKV-Spitzenverband requests significant discounts which can in the worst case lead manufacturers to withdraw from the German market.
Drugs with EMA orphan designation are eligible to submit a simplified dossier for G-BA benefit assessment and do not need to argue their added benefit against a comparator but rather provide evidence for the extent of the added benefit.
Justin Stindt Consultants is your agency for an optimal AMNOG dossier. Our team of experienced Consultants and experts will support you with a benefit assessment dossier meeting the G-BA and IQWiG requirements and bringing out the additional benefit of your product vs the appropriate comparator therapy.
Our agency has been selected by many pharma, biotech and medtech companies as service provider of choice to write the German P&R dossier / AMNOG dossier. Our company leverages German native speakers with extensive experience both in the industry and consulting agencies and a successful track record of P&R dossiers leading to sustainable Pricing & Reimbursement in Germany.
Reimbursement Pathways for Medical Devices in Germany
There are different pathways for the reimbursement of medical devices in Germany and Justin Stindt Consultants is the right agency to advise you on the optimal pathway for your medical device. Substance-based medical products can exceptionally be included in the supply of medicinal products according to § 31 SGB V (Positive list: Annex V of the directive).
A dossier for material medical devices has to be submitted so the medical device is included in Annex V.
Justin Stindt Consultants: Your Partner in German Market Access – Expert Consulting on Pricing & Reimbursement Dossiers

Justin Stindt Consultants is your agency to advise and support you with the application and the preparation of the benefit dossier for drugs and medical devices.
Our commitment lies in delivering HTA submissions that excel in written excellence, technical precision, and the art of weaving clear, captivating narratives. Our goal is to ensure that each submission forms a cohesive, integrated, evidence-based story, moving away from a mere assortment of distinct clinical and health-economic elements typically found in an evidence package.
Justin Stindt Consultants is your global agency with the right experts in writing AMNOG dossiers. Our services include (not limited to):
- Comprehensive project planning with clear timelines and milestones
- Strategic review of drug positioning
- Dossier writing, both German original and English translations (typically module 1)
- Dossier validation with Key Opinion Leaders and ex-members of the G-BA or IQWiG
- Submission process guidance and support
- Support with the written commentary procedure
- Hearing preparation and support
- Strategic advice and participation in price negotiations and if required arbitration process
- Effective project management and communication throughout the process
Our team of experts has extensive experience in providing our clients with tailored support to provide access to German patients as soon as possible at sustainable pricing and reimbursement conditions. If you are looking for a vendor to support your German Pricing & Reimbursement dossier, select a service provider that will go the extra mile to enable HTA endorsement and sustainable pricing for your product. More than a mere supplier, Justin Stindt Consultants can provide full service support including engagement with the authorities and price negotiations.
Let Justin Stindt Consultants be your trusted agency partner in unlocking pricing & reimbursement (P&R) opportunities for your product in Germany.
Contact our specialists today to learn how we can tailor our services to meet your unique needs and make your pricing & reimbursement goals a reality.
Meet one of our Pricing and Reimbursement dossiers Germany experts

Get in touch with our expert
CLIENT TESTIMONIALS








