Welcome to Justin Stindt Consultants, your trusted agency specializing in Joint Clinical Assessment (JCA) in the European Union (EU). As the European Community embarks on a new pan-European HTA process, the JCA aims to harmonize the assessment of innovative therapies across member states of the European Economic Community. Our global team of experts and consultants are proficient in navigating this transformative landscape, ensuring that your applications meet the rigorous standards set by the Health Technology Assessment Coordination Group (HTACG). As an international consulting agency with a successful track record in national P&R dossiers and pan-EU projects, we emphasize the importance of articulating the added benefit of your product for successful market entry in the EU. Rely on Justin Stindt Consultants as your partner in unlocking the potential of your innovative medical solutions in the European Union’s evolving market access framework.
Joint Clinical Assessment (JCA) in the EU: Challenges and Opportunities for Pharmaceutical Companies in the New Pan-European HTA Process
The Joint Clinical Assessment (JCA) process in the European Union represents a new pan-European HTA process progressively being implemented from 2025 onwards. While many hope that this will mean a significant step towards enhancing the collaborative evaluation of innovative therapies across member states, there are still concerns in the industry that this will lead to a duplication of efforts on the EU and national level. JCA seeks to streamline and harmonize the assessment of new treatments, aiming to optimize patient access to cutting-edge medicines and medical devices while ensuring cost-effectiveness and patient safety. Justin Stindt Consultants is an agency specialized in EU (JCA) and national HTA dossiers. Our experienced team of consultants and experts will help you to articulate the added benefit of your product in the JCA dossier.
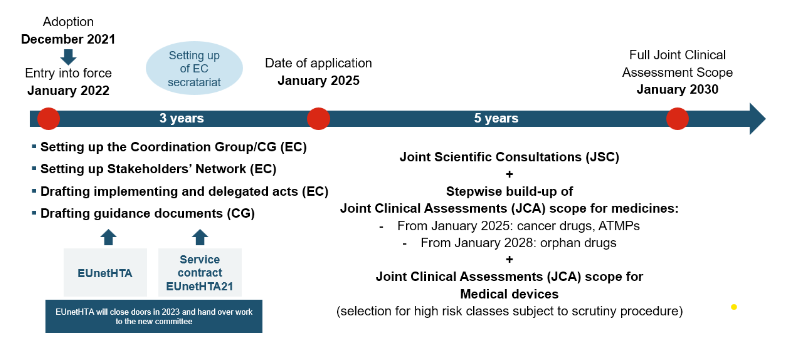
HTA Regulation – Timeline of Implementation and Market Access
The Promise of Joint Clinical Assessment (JCA) in the EU: A Faster and More Efficient Process
JCA will be conducted by the Health Technology Assessment Coordination Group (HTACG), which was established by the regulation on Health Technology Assessment (HTAR). The subgroup for Joint Clinical Assessments will assist the HTACG. The Coordination Group brings together various national health technology assessment (HTA) bodies and experts from different EU countries to jointly evaluate the clinical data and evidence supporting a particular therapy. By pooling resources and expertise, JCA aims to offer an efficient approach to assess the clinical and economic value of innovative treatments, reducing redundant evaluations and speeding up decision-making processes.
Challenges of Joint Clinical Assessment (JCA) in the EU: catering to the needs of very different healthcare systems
 Challenges in the JCA process arise from the diversity of European healthcare systems, treatment priorities, and patient populations among EU member states. Harmonizing criteria and aligning evaluation methods can be complex, requiring robust communication and cooperation among participating countries. Additionally, differing interpretations of clinical data and evidence may emerge, necessitating open dialogue and consensus-building during the assessment. A key challenge is the alignment on the PICOs [PICO = Population, Intervention, Comparator(s), Outcomes(s)], where member states can have very different expectations depending on their healthcare system and available set of comparators for example.
Challenges in the JCA process arise from the diversity of European healthcare systems, treatment priorities, and patient populations among EU member states. Harmonizing criteria and aligning evaluation methods can be complex, requiring robust communication and cooperation among participating countries. Additionally, differing interpretations of clinical data and evidence may emerge, necessitating open dialogue and consensus-building during the assessment. A key challenge is the alignment on the PICOs [PICO = Population, Intervention, Comparator(s), Outcomes(s)], where member states can have very different expectations depending on their healthcare system and available set of comparators for example.
For pharmaceutical and biotech companies, both big and small, the Joint Clinical Assessment (JCA) process in the European Union can be both a challenge and a game-changer.
The JCA process in the European Union marks a significant stride towards collaborative evaluation of innovative therapies, aiming to streamline assessments across member states. This initiative offers potential benefits in terms of patient access, cost-effectiveness, and decision-making efficiency. However, challenges arise from diverse healthcare systems and differing interpretations of data.
For pharmaceutical companies wishing to enter the market, adapting to this approach is crucial; small companies must engage actively with local HTA bodies and refine methodologies for advanced products, while larger companies need to align regional and local strategies to ensure the effective implementation and adoption of JCA reports. In sum, JCA holds promise for enhancing evaluation processes while demanding adaptability and cooperation from both industry and regulatory bodies. Companies looking for a supplier to support with JCAs can find in Justin Stindt Consultants a trusted service provider, leveraging the expertise of our network of experts and successful track record of national P&R dossiers and pan-EU projects
Joint Clinical Assessment (JCA) Timelines and Submission Modalities
The JCA will be published at the latest 30 days after the EPAR release. As the JCA report is set to be published just 30 days after the CHMP decision, national procedures may start very rapidly
Companies will have to submit a dossier 45 days before CHMP opinion. Dossier specifications are determined in REGULATION (EU) 2021/2282: Annex I for medicinal products, and Annex II for medical devices and in vitro diagnostic medical devices.
The outcome of the JCAs will be published 30 days after the CHMP decision so it can influence national procedures.
Justin Stindt Consultants: An Expert Consulting Agency Specializing in EU Pricing & Reimbursement Dossiers / JCA

Joint Scientific Consultation (JSC): Pan-European Scientific Advice
Pharma, biotech and medtech companies will also be able to request a Joint Scientific Consultation (JSC) from the Coordination Group when the clinical studies are being planned. For more information visit our EU Joint Scientific Consultation page.
Justin Stindt Consultants is your agency with the right experts in supporting the preparation of JCA dossiers. Our services include (not limited to):
- Comprehensive project planning with clear timelines and milestones
- Strategic review of drug positioning
- Dossier writing, both in English and other EU languages
- Dossier validation with Key Opinion Leaders and payers
- Submission process guidance and support
- Strategic advice and participation in national level price negotiations
- Effective project management and communication throughout the process
At Justin Stindt Consultants, our team of experts has extensive experience in providing our clients with tailored support to provide access to EU patients as soon as possible at sustainable pricing and reimbursement conditions.
Let Justin Stindt Consultants be your trusted agency partner in unlocking pricing & reimbursement (P&R) opportunities for your product in the EU.
Contact us today to learn how our firm can tailor our services to meet your unique needs and make your pricing & reimbursement goals a reality.
Meet one of our Pricing and Reimbursement dossiers EU experts

Get in touch with our expert
CLIENT TESTIMONIALS