Accelerating your Market Access for innovative medical technologies with the ‘Trial upon application’ (Antragsgesteuerte Erprobung) pathway in Germany
‘Trial upon application’ (Antragsgesteuerte Erprobung), a process initiated by the G-BA, holds the promise of streamlining market access for innovative medical technologies in the Federal Republic of Germany. Medtech and diagnostics companies can leverage this pathway to submit applications, which are meticulously evaluated by the G-BA. Successful applications then pave the way for the G-BA to design and execute tailored clinical trials.
Through this pathway, innovative medical technologies can be made available ahead of regular reimbursement decisions which can take many years. Therefore, it allows patients to benefit from the medical technologies’ potential therapeutic advantages sooner. Also, generating positive data through this pathway enhances companies’ chances of obtaining reimbursement for their products, increasing market entry and adoption potential.

Justin Stindt Consultants is a leading consulting agency specialized in the trial upon application pathway. Our dedicated team of consultants and specialists will help you articulate the added benefit of your product in the trial upon application dossier. The overall process is complex, but with our expert team by your side, your primary responsibilities end after the application submission.
Gain market access in Germany prior to having a marketing authorization, thanks to the ‘Compassionate Use’ (Härtefall) program
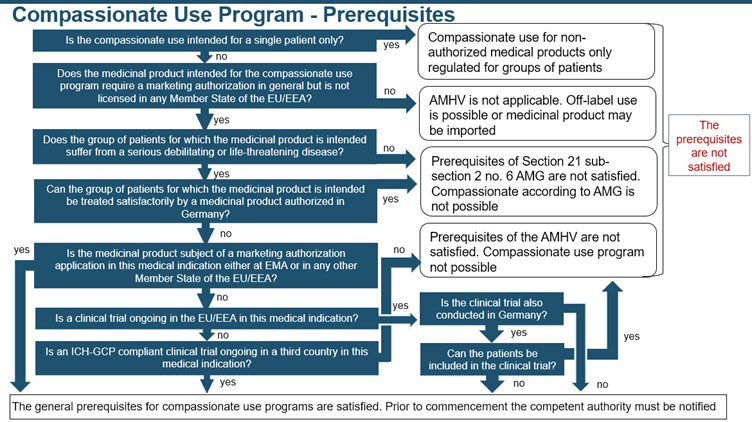
- PEI is the competent authority for Sera, Vaccines, Blood preparations, Tissues and tissue preparations, Allergens, Advanced therapy medicinal products, Xenogenic medicinal products, and Genetically engineered blood components
- BfArM is the competent authority for all other medicinal products
This program facilitates the early availability of the drug before obtaining marketing authorization, enabling patients to access its benefits sooner. Furthermore, the additional support provided through the generation of positive data, can strengthen the company’s case for securing marketing authorization. The following decision tree is followed to assess whether the prerequisites are met:
If your company is considering this route, Justin Stindt Consultants stands ready to assist. Our consultants bring valuable expertise in preparing pharmaceutical companies for compassionate use notifications. Navigating the various documents required can be complex, but with our experienced team, you will have dedicated support every step of the way.
Our agency has been selected by many pharma, biotech and medtech companies as service provider of choice to write the German early access applications. Our company leverages German native speakers with extensive experience both in the industry and consulting agencies and a successful track record of early access dossiers leading to early access in Germany.
ENHANCING MARKET ACCESS WITH JUSTIN STINDT CONSULTANTS: EXPERTISE IN ANALOGUE RESEARCH PRICE BENCHMARKING AND GERMAN HEALTHCARE CONSULTATIONS

At Justin Stindt Consultants, we understand the intricacies of this complex process and are here to support you every step of the way. Before embarking on this journey, we recommend an advice meeting with the G-BA in order to assess the evidence requirements and scope out the chances of success. Our team of experts has extensive experience in creating advice requests in accordance with Article 137e SGB V. Our consulting firm provides a comprehensive assessment of requirements, evidence-based support for your applicant’s position, and in-depth preparation and participation in G-BA consultations.
By partnering with Justin Stindt Consultants as your agency, you gain access to our wealth of knowledge and expertise, empowering you to confidently submit your applications and navigate the intricacies of the ‘Trial upon application’ (Antragsgesteuerte Erprobung) pathway. Your primary responsibilities end after application submission; we take care of the rest. Let us be your trusted agency in accelerating your market access journey in Germany.
If you are looking for a vendor to support your German early access programmes, select a service provider that will go the extra mile to enable early access for your product. More than just a mere application supplier, Justin Stindt Consultants will partner with you and can provide full-service support including engagement with the authorities.
Take the first step towards securing market access for your innovative medical technologies. Contact us today to learn how we can tailor our services to meet your unique needs and make your reimbursement goals a reality.
Meet one of our Early Access Programs Germany experts

Get in touch with our expert
CLIENT TESTIMONIALS