France’s Reimbursed Early Access Programs (EAP) allow for reimbursement of new drugs and medicines before traditional reimbursement making it a very attractive option for pharma and biotech companies with medicines in the early stages.
336 products and 218 unique active substances (inns) reimbursed
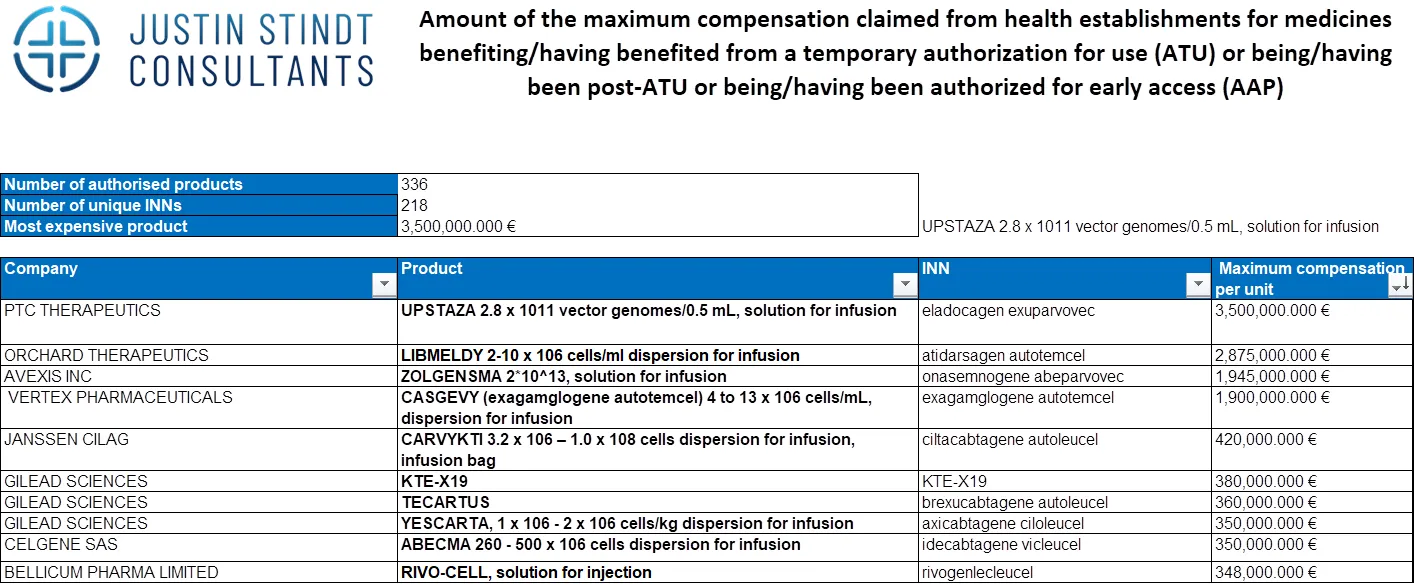
As of February 2024, there are 336 products covered in the early access scheme list, of which there are 218 unique INNs. The presence of multiple products under the same INN is attributable to variations in formulations or indications. This extensive list reflects the ongoing efforts to make a wide range of innovative treatments available to patients who need them most, and underlines the attractiveness of the French market for pharma and biotech firms.
Reimbursement can reach millions of euros per patient
The most expensive product listed in the early access program in 2024 is Upstaza, a gene therapy medicine for a rare disease that affects the nervous system, leading to symptoms such as developmental delays, weak muscle tone and inability to control the movement of the limbs (AADC deficiency). The price of Upstaza, covered by the statutory health insurance, is €3,500,000. Notably, the top ten most expensive products in the list are all cell and gene therapies, indicating a potential shift towards personalized and precision medicine, promising improved outcomes for patients with complex and/or rare conditions (see in picture below).
Overall, the 2024 list includes innovative treatments, including cell and gene therapies such as PTC Therapeutics’ Upstaza (eladocagen exuparvovec), cancer therapies like AstraZeneca’s Imjudo (tremelimumab), and other innovative treatments like MaaT’s microbiome-based product MaaT013. Justin Stindt Consultants has compiled and translated to English the complete list of the products reimbursed under the ATU/AAP scheme, please contact us for a free copy.
JSC Expertise
Justin Stindt Consultants is a specialized market access consulting firm offering bespoke market access services. Our team of experts has extensive experience in AAP and AAC feasibility assessments and dossier preparation, ensuring that our clients are well-prepared for seamless application submissions. Our experts have assisted numerous clients in establishing early access programs in France, including the former ATU and the current AAP and AAC programs. Our agency has been the preferred service provider for many pharmaceutical and biotechnology companies seeking assistance with crafting French early access applications. We employ French native speakers with extensive experience in the industry and consulting agencies, and we have a proven track record of delivering successful dossiers that pave the way for early access in France.
Stay updated
We are closely monitoring the ATU-AAP tables of maximum compensation which are updated typically three times a year and will keep our clients informed of the latest changes. For more detailed insights and updates, visit our blog regularly.
Why choose us?
- Proven Expertise: With years of experience in global pricing and reimbursement, our team offers unparalleled expertise and strategic insights.
- Tailored Solutions: We understand that each client and market is unique, and we provide customized solutions that address specific needs and challenges.
- Comprehensive Support: From initial strategic advice to implementation and beyond, we offer end-to-end support to ensure your success.
- Regulatory Mastery: Our deep understanding of regulations, requirements and processes, including extensive experience with HAS, positions us to effectively advocate for your products.
Partner with Justin Stindt Consultants to leverage our expertise and achieve your market access goals with confidence.





