January 12th marked the launch of Joint Clinical Assessment (JCA), a key milestone in the European Union’s Health Technology Assessment (EU HTA) process. This transformative initiative aims to harmonize drug and technology assessments across member states, promising faster patient access to innovative therapies.
From January 12th, the new JCA and JSC procedures will apply to marketing authorisation applications for oncology drugs and advanced therapy medicinal products (ATMPs). The extension to orphan medicines is planned for January 2028, with other medicinal products to be included in 2030. Some high-risk medical devices will also be assessed as of 2026.
Considerable advances were made in publishing the implementing acts and guidance documents over the past year, particularly in Q4 2024, when 14 new documents were released. The tables below show the current tracking of what has been published. Most documents pertaining to medical devices and IVDs are yet to be published, likely because these will not be eligible for the EU HTA earlier than 2026.
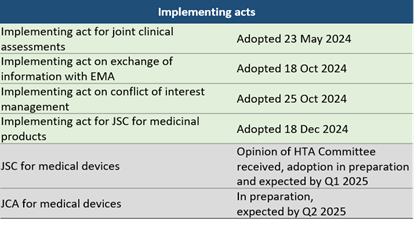
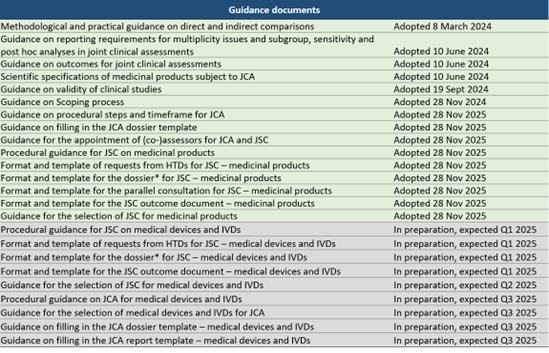
Clearer scoping procedure
One of the key guidelines recently published focuses on the scoping process, clarifying how PICOs (Population, Intervention, Comparator, Outcomes) will be consolidated across member states.
Anticipating the PICOs that will be included in the assessment scope is critical, as health technology developers (HTDs) must prepare their dossiers within tight deadlines—100 days for the standard procedure or just 60 days for the accelerated procedure.
The new guidance document provides a practical example of how member states will consolidate PICOs into a unified scope. Interestingly, the Comparator (C) in the PICO framework may or may not be licensed in the EU or a specific member state but can still be chosen as the comparator. This raises questions about potential disparities in national assessments, as for example in Germany G-BA can only select licensed therapies as comparator.
Detailed JCA and JSC timelines
The recently published guidance outlines the timeframes for the two key processes, JSC and JCA.
Key timeframes include:
- JSC: A 4.5-month total process, with the discussion meeting held around 60 days after the health technology developer submits the briefing package.
- JCA (Standard Procedure): A 406-day timeline for products undergoing the EMA’s standard approval process, with 100 days HTDs have to submit the dossier following the scope notice
- JCA (Accelerated Procedure): A 266-day timeline for products following the accelerated EMA procedure, with 60 days HTDs have to submit the dossier following the scope notice
These structured timelines emphasize the need for developers anticipate the likely PICOs and start preparing for and engaging with the processes well in advance.
Resource problems for member states?
A 2024 report (PDF) by Swedish authorities (TLV, SBU, Medical Products Agency) highlights the complexities of the EU HTA framework and quantifies the additional resources needed for national agencies to participate effectively. Key Findings from Sweden’s Analysis.
- Additional Staffing Needs for TLV:
- 4.5 additional FTEs for PICO development.
- 7 additional FTEs to act as assessors.
- 1 additional FTE for participation in EU working groups (Coordination Group, JCA and JSC subgroups, methodology subgroup, horizon scanning subgroup).
- Estimated Budget Impact:
- The total additional cost is projected to be approximately SEK 16 million.
- SBU Staffing Needs:
- The SBU expects to require 4-5 additional FTEs to manage EU joint HTA responsibilities.
- Initial remuneration for assessor/co-assessor roles is expected to cover only one-third of the costs.
- Limited Assessment Capacity:
- Sweden will only have the capacity to act as an assessor for one drug in 2025—raising concerns about how other EU countries will manage the transition.
This raises an important question: how will other member states handle the upcoming workload?
Overall, January 12th marks the start of a new era, bringing high hopes for improving patient access to new technologies. At the same time, this period is characterized by uncertainty and novelty, requiring stakeholders to act with agility to ensure success. There is also concern among the industry about likely duplication of efforts, at least in the initial stages of JCA.
Stay updated
We are organising a webinar on the topic of the JCA of February 6th 2025. If you would like to participate please do not hesitate to reach out to us or click the button to sign up.
Until all details on the process are unveiled, significant uncertainty persists and concerns of a duplication of HTA processes on EU and member state level. Our expert consultants are closely tracking all EU HTA related news and changes relevant to our pharma, biotech and medtech clients. For more detailed insights and updates, visit the JSC blog regularly.
Justin Stindt Consultants’ Expertise
At Justin Stindt Consultants, we specialize in:
- JCA dossiers, PICO assessments
- Joint Scientific Consultations (JSC)
- European Pricing & Reimbursement: Providing strategic advice on pricing and reimbursement pathways, analogue research, and price benchmarking.
- Health Technology Appraisal (HTA) Scientific Advice: Offering expert guidance to optimize EU and global HTA processes and meet regulatory requirements.
- Payer Advisory Boards: Facilitating meaningful dialogue between manufacturers and payers to align product value propositions with payer expectations.
- Comprehensive Market Access Training: Equipping teams with the necessary skills and knowledge to succeed in the dynamic healthcare landscape.
We are committed to helping pharmaceutical, biotech and companies navigate complex global markets effectively and achieve their market access goals.





