The implementation of the Joint Clinical Assessment (JCA) in the European Union represents a notable step forward in assessing innovative therapies across the member states. The main objective of the JCA is to unify and streamline the evaluation processes for new treatments, thereby improving patient access to state-of-the-art medical solutions while maintaining cost-efficiency and ensuring patient safety. Nonetheless, the industry has expressed concerns over the potential for duplicative evaluations at both the EU and national levels due to some unresolved details about the process and its fast-approaching roll-out.
Overview of the JCA
The JCA framework involves cooperative efforts among national health technology assessment (HTA) bodies and specialists from across the EU. These parties work together to review clinical data and evidence related to specific therapies. The intent is that by combining resources and expertise, the JCA will provide a more streamlined and effective method for assessing the clinical and economic value of new treatments. This cooperative approach aims to eliminate duplicate assessments, accelerate decision-making processes, and establish uniform standards throughout the member states.
JCA implementing regulation
The JCA Implementing Act for Joint Clinical Assessments, adopted on 23rd of May 2024, outlines crucial process steps and timelines that impact all parties involved, including the health technology developers (HTD, i.e. the manufacturer).
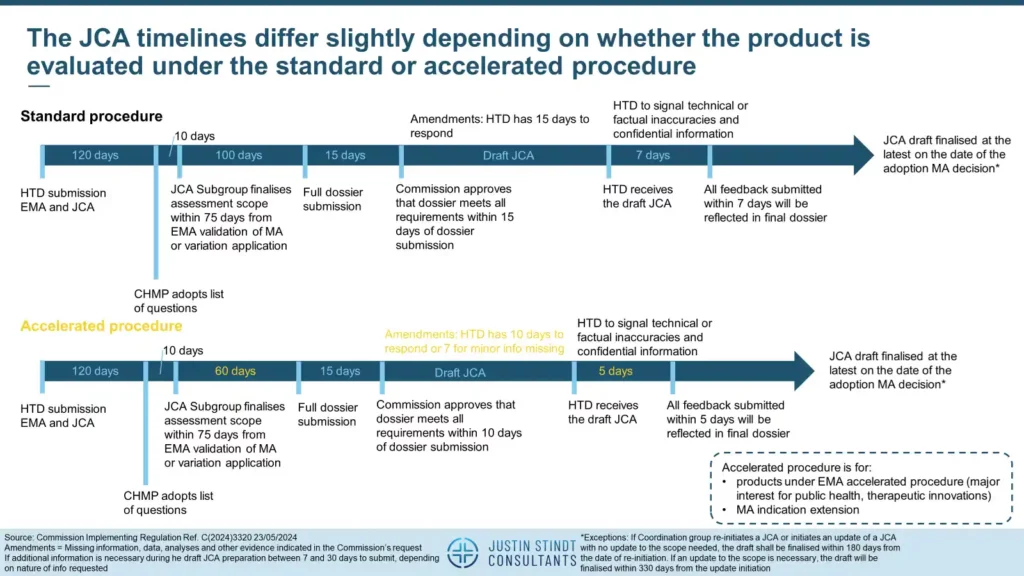
According to the document, the JCA runs in parallel to the EMA Marketing Authorization (MA) procedure. The JCA timelines differ slightly depending on whether the product is evaluated under the standard or accelerated procedure.
JCA for products under the standard procedure
For products under the standard procedure, the HTD submits the EMA and relevant information for developing the assessment scope of a joint clinical assessment at the same time. The Committee for Medicinal Products for Human Use (CHMP) adopts their list of questions up to 120 days after the dossier submission, at which point the JCA subgroup has 10 days to finalize the assessment scope. Further, the JCA Subgroup finalises the assessment scope within 75 days from the day on which the EMA validates MA application or MA variation. Upon request of the HTD, the HTA secretariat will invite the HTD to an assessment scope explanation meeting with the JCA Subgroup. The meeting will take place no later than 20 days from the day on which the JCA Subgroup finalises the assessment scope. One hundred days later, the HTD is to submit the full dossier. After a 15-day completeness check delay, the JCA assessment starts, with the final version being communicated no later than the date of the adoption of the MA decision.
JCA for products under the accelerated procedure
The accelerated procedure is designated for products under EMA accelerated procedure, that is, for products that have a major interest for public health and/or therapeutic innovations, as well as for cases of MA indication extension. The activities are the same as in the standard procedure except that for some processes the timelines are shortened. Thus, the HTD has 60 days instead of 100 days to submit the full dossier following the scope assessment. For amendments or minor comments during the draft JCA assessment, the HTD has 10 days (or 7 if minor information is missing) instead of 15 days to respond. Lastly, the final check after the HTD receives the draft JCA is shortened from 7 to 5 days. As in the standard procedure, the final JCA is finished no later than the MA decision adoption.
Key differences between the Draft and the adoption document:
Article 10 Finalisation of the assessment scope
- The JCA Subgroup shall finalise the assessment scope at the latest 10 days (as opposed to 20 in the draft) after the Committee for Medicinal Products for Human Use adopts its list of questions.
The JCA Subgroup shall finalise the assessment scope within 75 days (as opposed to 100 in the draft) from the day on which the European Medicines Agency validates the application for a marketing authorisation or a variation to the terms of an existing marketing authorisation, where:
(a) the application for a marketing authorisation for a medicinal product is assessed under the accelerated procedure referred to in Article 14(9) of Regulation (EC) No 726/2004; or
(b) the joint clinical assessment is conducted for a medicinal product referred to in Article 7(1), point (b), of Regulation (EU) 2021/2282, for which a variation to the terms of an existing marketing authorisation is of the type referred to in point 2(a) of Annex II to Commission Regulation (EC) No 1234/2008 and corresponds to a new therapeutic indication.
Article 11 Assessment scope explanation meeting
Upon request of the health technology developer, the HTA secretariat shall invite the health technology developer to an assessment scope explanation meeting with the JCA Subgroup. The meeting shall take place no later than 20 days (as opposed to 30 in the draft) from the day on which the JCA Subgroup finalises the assessment scope.
The JCA implementation rolling plan
The implementation rolling plan contains a list of key activities that the Commission has carried out or intends to carry out as it prepares for the rolling out of the new HTA regulation. The last update to the document as of September 2024 was in June. The plan is subject to regular updates to keep relevant bodies and stakeholders up to date on recent JCA/JSC-related developments. Importantly, the latest update of the implementation rolling plan of Regulation (EU) 2021/2282 on HTA points to the following expected adoption timelines for JCA and JSC:
- Joint Scientific Consultations for medicinal products: Q3 2024
- Joint Clinical Assessments for medical devices: Q4 2024
- Joint Scientific Consultations for medical devices: Q4 2024
Until all details on the process are unveiled, significant uncertainty persists and concerns of a duplication of HTA processes on EU and member state level. For more information on the JCA process, follow Justin Stindt Consultants’ coverage of news from relevant EU authorities.
Justin Stindt Consultants’ Expertise
At Justin Stindt Consultants, we specialize in:
- JCA dossiers, PICO assessments
- Joint Scientific Consultations (JSC)
- European Pricing & Reimbursement: Providing strategic advice on pricing and reimbursement pathways, analogue research, and price benchmarking.
- Health Technology Appraisal (HTA) Scientific Advice: Offering expert guidance to optimize EU and global HTA processes and meet regulatory requirements.
- Payer Advisory Boards: Facilitating meaningful dialogue between manufacturers and payers to align product value propositions with payer expectations.
- Comprehensive Market Access Training: Equipping teams with the necessary skills and knowledge to succeed in the dynamic healthcare landscape.
We are committed to helping pharmaceutical, biotech and companies navigate complex global markets effectively and achieve their market access goals.





