The EU Health Technology Assessment Regulation (HTAR) represents one of the most significant shifts in the European health-innovation landscape in decades. Although it entered into force in 2022, its application began on 12 January 2025, starting with oncology products and ATMPs, before gradually extending to orphan medicines, medical devices, and IVDs in the following years.
The ambition is clear:
a single, coordinated EU-level system for HTA, reducing duplication across member states, harmonising methodologies and ultimately accelerating patient access to innovation.
Whether this vision becomes reality remains a matter of debate. Many manufacturers fear the reform might add requirements rather than streamline them. Yet, the reform is now fully mandatory — and its impact is already unfolding.
Two pillars define the new system:
- Joint Clinical Assessments (JCAs): EU-level evaluations of relative clinical effectiveness and safety.
- Joint Scientific Consultations (JSCs): Voluntary early dialogue with HTA bodies to optimise evidence-generation plans.
Where Do We Stand Now? A 2025 Update
As of 25 November 2025, nearly one year into the operational phase, the system is beginning to take shape:
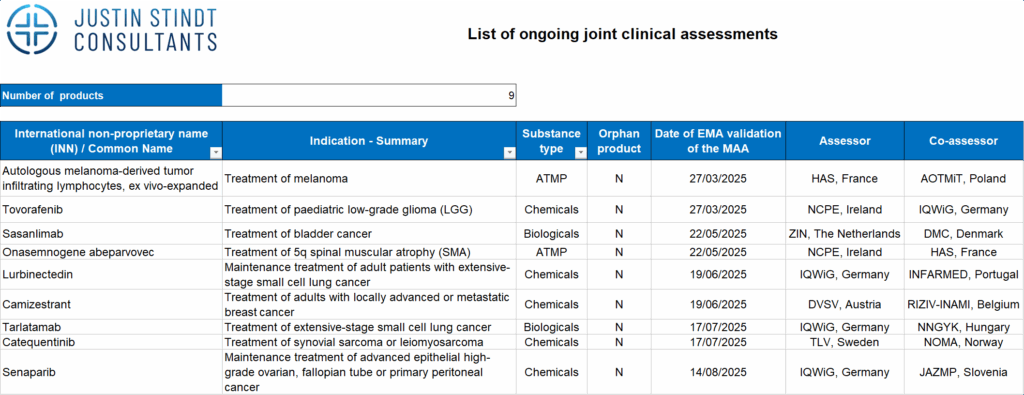
Joint Clinical Assessments
- Nine JCAs are currently ongoing, all for new oncology medicines and ATMPs1.
- The first JCA outcomes are expected in 2026, creating the first real test of how member states will apply “due consideration.”
Major 2025 Development: Implementing Regulation for Devices & IVDs
A key milestone came on 17 October 2025, when the European Commission adopted the Implementing Regulation2 detailing procedures and templates for JCAs of medical devices and IVDs.
How JCAs for Devices/IVDs Will Work
- Automatic Trigger:
A JCA is triggered once a device receives certification under MDR/IVDR. The HTA Secretariat is notified automatically. - Early Information Phase:
The HTA Secretariat invites the manufacturer (health technology developer) to submit early information about the product. - Scoping (PICO) Timeline:
The JCA Subgroup must finalize the scope (i.e. confirm the PICO and key questions) within 60 days of receiving early information, or within 10 days of the Commission’s formal selection of the device for the JCA, whichever is later. - Optional Scope Explanation Meeting:
Manufacturers may request a meeting to clarify scope decisions, held within 20 days of receiving the scope. - Dossier Submission:
The manufacturer has 100 days to submit the full JCA dossier. - Assessment Timeline:
The JCA Subgroup has 165 days from dossier validation to deliver the joint assessment report.
This creates a structured but tight process, especially given that devices often enter markets with comparatively limited clinical evidence.
How Health-Technology Developers Should Prepare: Insights from Justin Stindt Consultants.
A successful JCA depends on anticipating the PICO well before the formal scoping phase begins. Developers will only have 100 days to prepare their dossier once the scope is final — far too short for major evidence-generation adjustments.
At Justin Stindt Consultants, we support clients through a proven, multi-step preparation strategy:
1. Early PICO Anticipation
We combine:
- Secondary research on national HTA precedents
- Primary payer research to identify expected national PICOs and clinical expectations
This consolidated view helps manufacturers avoid surprises during the formal JCA scoping phase.
2. JSC (Joint Scientific Consultation) Strategy
Early engagement with EU and national HTA bodies helps refine endpoints, comparators, and evidence generation plans.
3. Benchmarking Against Previous JCAs
As JCA reports become available in 2026 and beyond, analysing precedent cases will become essential for planning robust submissions.
4. Linking EU-Level Assessments to National Market Access
Although the JCA sets a unified EU view on clinical evidence, pricing and reimbursement decisions remain national.
Manufacturers must map:
- How each country interprets the JCA
- How it influences local P&R processes
- Whether additional national evidence will still be requested
This “EU → National” translation is critical for launch sequencing and value communication.
Upcoming: Webinar for Device and IVD Manufacturers
Health-technology developers preparing for future JCAs can join the HTACG Webinar “Webinar for HTDs of medical devices and in vitro diagnostic medical devices” on 12 December.
For asset-specific questions or JCA preparation support, our consultants are available for tailored guidance.
Why Work With Justin Stindt Consultants
Our team specialises in guiding pharmaceutical, biotech, medtech and diagnostic companies through the evolving European market access environment. Our expertise includes:
JCA Dossiers & PICO Assessments
Designing and delivering evidence packages aligned with EU HTA expectations.
Joint Scientific Consultations
Preparing high-impact interactions with HTA bodies at the EU level to optimise clinical plans.
Pricing & Reimbursement Strategy
Pathway definition, analogue research, price benchmarks and launch sequencing.
HTA Scientific Advice
Guiding clinical and economic strategy to meet national payer expectations.
Payer Advisory Boards
Facilitating strategic dialogue to refine value messaging and ensure payer resonance.
Our mission is to help health-technology developers navigate a complex, shifting EU landscape and achieve timely, sustainable market access.
Sources
[1] https://health.ec.europa.eu/health-technology-assessment/implementation-regulation-health-technology-assessment/joint-clinical-assessments_en
[2] https://eur-lex.europa.eu/legal-content/EN/TXT/?uri=OJ:L_202502086





