On July 4, 2024, the Bundestag (German Parliament) passed significant amendments to the country’s drug pricing and reimbursement laws as part of the new Medical Research Act (Medizinforschungsgesetz or MFG). The changes aim to bolster Germany’s position as a hub for medical research and ensure a level playing field in terms of confidential pricing compared to EU neighbours. The law still needs to go through the Bundesrat (the Federal Council), which is planned for September 27th. The entry into force is expected in October 2024. Here’s what you need to know.
Medizinforschungsgesetz Introduces Confidential Reimbursement Prices
One of the most debated provisions is the introduction of confidential net reimbursement prices. Until now, prices were publicly available in Germany. However, under the new law, pharmaceutical companies can keep the reimbursement prices agreed upon through the AMNOG process and price negotiations with GKV-SV confidential. This aims to prevent potentially unfavorable German prices from affecting pricing in other European markets through international reference pricing. In the past some manufacturers withdrew drugs from the German market for this reason.
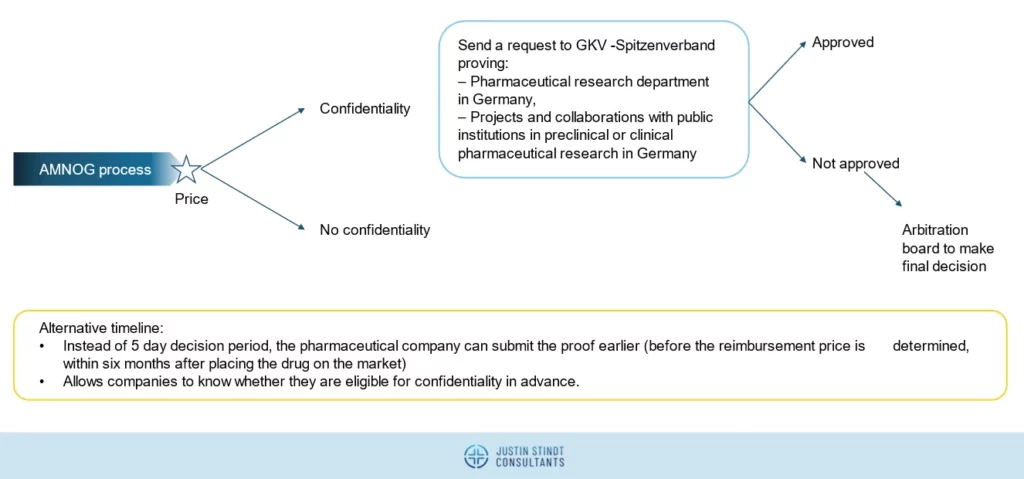
Requirements for confidential prices in Germany
Companies that opt for confidentiality will have to pay an additional 9% discount on the agreed AMNOG reimbursement price. Manufacturers have to request this within 5 days of agreeing the reimbursement amount with GKV-SV. Further, to be eligible for the confidentiality, companies must demonstrate local R&D activities in Germany (e.g., owning a pharmaceutical research department and conducting collaborations with public institutions).
This option is only available for drugs for which the AMNOG procedure is concluded until 30 June 2028 (the sunset clause). The new rules for confidential reimbursement prices are envisaged to apply from 1 January 2025.
Incentivising research in Germany through relaxed guardrails
The new law also incentivizes companies to conduct clinical trials in Germany. Drugs with at least 5% of clinical trial participants enrolled at German trial sites will benefit from relaxed pricing guardrails (from the GKV Financial Stabilization Act) during reimbursement negotiations.
Efforts are also underway to simplify the approval process for medicines and clinical trials. This includes improved cooperation between approval authorities, the harmonization of ethics committees, and a reduced processing time of 26 days for trials conducted solely in Germany.
Confidential Prices Won’t Be Fully Confidential
The law allows certain parties, such as healthcare institutions, importers, and entities purchasing the drug, to access the confidential price. Furthermore, information on the cost-effectiveness of drugs, including confidential reimbursement amounts, will be available to doctors to ensure that drugs are prescribed economically.
Next Steps and Implementation
The Medical Research Act is expected to pass the Federal Council (Bundesrat) in October 2024, with many provisions, including confidential reimbursement pricing, taking effect on January 1, 2025. Pharmaceutical companies should assess how these changes will impact them, and adequately prepare their market access strategies for the new changes that are on the horizon.
Stay updated
Justin Stindt Consultants are closely monitoring all developments related to the Medizinforschungsgesetz and will keep our clients informed of the latest changes. For more detailed insights and updates, visit our blog regularly.
Justin Stindt Consultants can help you with German Market Access
Justin Stindt Consultants is your consulting firm with experts who have extensive experience with Market Access in Germany. Our strong presence in the German market means that we can respond to the new law with agility and help you launch your asset seamlessly despite the changes and uncertainty.
Our consultancy has helped many clients by engaging the G-BA for scientific advice meetings and developing benefit assessment AMNOG dossiers. Our consultants also support price negotiations. For medical devices and digital health apps, our agency supports dossiers and negotiations. Many of our consulting firm’s clients have found Germany the place to start for Market Access in Europe and decided to enlist our agency as partner of choice given our footprint and experience in the German market.
Contact our experts today to learn how our agency can tailor our services to meet your unique needs to enable Market Access in Germany.
Why choose us?
- Proven Expertise: With extensive experience in global pricing and reimbursement, our team offers unparalleled expertise and strategic insights.
- Tailored Solutions: We understand that each client and market is unique, and we provide customized solutions that address specific needs and challenges.
- Comprehensive Support: From initial strategic advice to implementation and beyond, we offer end-to-end support to ensure your success.
- In-depth knowledge of regulations: Our deep understanding of regulations, requirements and processes, including extensive experience with the G-BA, positions us to effectively support your products.








